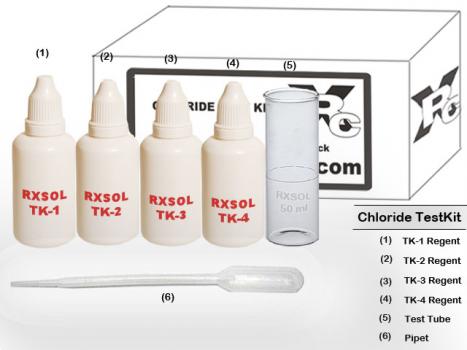
Chloride LMP Test Kit contains everything needed to measure chloride in boiler water for low-pressure (0-32 bar and medium-pressure (32-60 bar) boiler. Ready stock at Kolkata, Mumbai, Chennai - Ennore, Visakhapatnam, Kandla, Fujairah - Dubai, Barka Muscat.
PROCEDURE for test ( STEP WISE ):
- Measure 15 ml of SAMPLE WATER in graduated TEST TUBE / CYLINDER
- Add 1-2 drops of RXSOL TK1 and mix with the stirring rod , If sample turns red / PINK then follows 3rd step otherwise if sample remains colourless proceed to step 4.
- Add RXSOL TK2 drop by drop , mixing with the stirring rod until colour just disappears.
- Add 3 drops of RXSOL TK3 and mix with stirring rod , the sample will turn YELLOW.
- AddRXSOL TK4 carefully by counting drop by drop and mixing throughly until a light reddish / pink / brown colour develop.
RESULT : Each DROPS is equivalent to 8 PPM of CHLORIDES .
Small amount of sample is collected in a test tube and an indicator reagent is added to the sample. Use the dropper bottle, the titrant, until the color changes. To calculate the concentration just multiply the number of drops required by the titrant by a simple factor given in the test kit manual.
VIDEO HELP : https://www.youtube.com/watch?v=p_IgKoO49iE
|
TK4 Drops |
Chloride value in |
PPM |
|
1 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
8 |
|
2 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
16 |
|
3 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
24 |
|
4 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
32 |
|
5 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
40 |
|
6 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
48 |
|
7 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
56 |
|
8 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
64 |
|
9 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
72 |
|
10 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
80 |
|
11 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
88 |
|
12 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
96 |
|
13 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
104 |
|
14 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
112 |
|
15 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
120 |
|
16 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
128 |
|
20 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
160 |
|
24 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
192 |
|
26 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
208 |
|
28 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
224 |
|
30 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
240 |
|
32 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
256 |
|
34 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
272 |
|
36 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
288 |
|
38 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
304 |
|
40 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
320 |
|
42 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
336 |
|
44 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
352 |
|
46 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
368 |
|
48 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
384 |
|
50 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
400 |
|
55 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
440 |
|
60 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
480 |
|
70 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
560 |
|
90 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
720 |
|
100 |
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> |
800 |
CHLORIDES Content up to 100 PPM level s are acceptable , in low pressure boiler , while more then 300 PPM level should be reduced by increased blowdown . Where as chlorides levels are very high the quality of the feed water should be checked in view to possible saline contamination.
OFFER :::---
Do not forget to ask JUMBO PACK OFFER ( TK - extra pack )
BUY 2 - GET 2 FREE TK extra pack
REAGENT & APPARATUS:
Reagent: RXSOL TK 1 , TK 2 , TK 3 , TK4 . Apparatus : TEST TUBE , Titrator or DROPPER.
VIDEO Help : https://www.youtube.com/watch?v=p_IgKoO49iE
| TK4 Drops | Chloride Value (PPM) |
|---|---|
| 1 | 8 |
| 2 | 16 |
| 3 | 24 |
| 4 | 32 |
| 5 | 40 |
| 6 | 48 |
| 7 | 56 |
| 8 | 64 |
| 9 | 72 |
| 10 | 80 |
| 11 | 88 |
| 12 | 96 |
| 13 | 104 |
| 14 | 112 |
| 15 | 120 |
| 16 | 128 |
| 20 | 160 |
| 24 | 192 |
| 26 | 208 |
| 28 | 224 |
| 30 | 240 |
| 32 | 256 |
| 34 | 272 |
| 36 | 288 |
| 38 | 304 |
| 40 | 320 |
| 42 | 336 |
| 44 | 352 |
| 46 | 368 |
| 48 | 384 |
| 50 | 400 |
| 55 | 440 |
| 60 | 480 |
| 70 | 560 |
| 90 | 720 |
| 100 | 800 |
Estimation of chloride as silver chloride in a given solution
To estimate chloride as silver chloride in the given solution of sodium chloride or hydrochloric acid.
Principal: The estimation of a soluble chloride is carried out by precipitating it as silver chloride in the presence of nitric acid using silver solution as a precipitating agent.
Cl- + Ag+ AgCl
The white curdy precipitate of silver chloride is filtered, washed with dil. nitric acid, dried and weighed.
Solution: Prepare a solution by dissolving 4.9g or 6.53 g or 8.157 g of sodium chloride per litre. 25 ml of such a solution will yield 0.3, or 0.4 or 0.5 g of AgCl residue respectively.
Reagents.
(a) Precipitating reagent : It is 0.1 N AgNO3 solution which is prepared by dissolving 4.22 g of silver nitrate (A.R.) in 250 ml of distilled water. (b) Washing solution : (i) One is 0.1N HNO3 which is prepared by adding 3 ml of conc. nitric acid (A.R.) to 497 ml of water, making the total volume 500 ml (ii) Other is 0.01N HNO3 which is prepared by adding 10 ml of 0.1N HNO3 (prepared as above) to 90 ml water making the total volume as 100 ml.
Procedure
(i) Precipitation : Measure out 25 ml of the given solution in a 500 ml beaker. To this add 50 ml of distilled water and 1 ml of conc. nitric acid (A.R.). Warm the solution at 700C and add 0.1N silver nitrate solution slowly with vigorous, constant stirring until precipitation is complete. Avoid a large excess of silver nitrate and carry out all operations in subdued light. Now wrap the beaker with a thick brown paper. Keep the beaker on a water bath for few minutes until the ppt. settles down and the supernatant liquid becomes clear. Allow the solution to cool a little and test the supernant liquid with a little more silver nitrate solution to ensure that precipitation is complete. If a ppt. appears, add more silver nitrate till the supernatant liquid yields a ppt. Again heat the beaker to 70 – 800 C
and stir constantly to coagulate the ppt. In case no ppt. appears, there is no need for adding excess of silver nitrate. After doing this test for complete precipitation, heat the beaker on a water bath. Cover the beaker with a watch glass and keep it about 25 minutes. Cool the beaker and keep it in dark for 1 hour to allow the ppt. to settle down.
(ii) Filtration and washing : Now transfer the supernatant liquid through a sintered glass crucible G – 3 previously cleaned, heated in an oven and weighed after cooling in a desiccator. Wash the ppt. by decantation 3 – 4 times with 0.1N HNO3 and the transfer the ppt. from the beaker to the crucible using a policeman to remove any adhering ppt. from the sides of the beaker. Now wash the ppt. in the crucible by 0.1N HNO3 until washings are free from Ag+ ions (when few-drops of dil. HCl are added to 10 – 15 drops of the filtrate, no turbidity should appear). Finally, wash
the ppt. with cold distilled water twice to free it from NO-
(iii) Drying and weighing : Dry the ppt. by keeping the crucible in an electric oven at 110 – 1200C for an hour. Allow the crucible to cool in a desiccator and weight it. Repeat the heating and cooling until constant weight is obtained.
Calculations
The constant weight of the crucible = a g
The constant weight of the crucible AgCl = b g
Weight of AgCl = (b – a ) g
Now AgCl ( 143.34 ) = Cl- ( 35.46 ) = NaCl ( 58.46 )
Here Simple Calculation is : 143.34 g of AgCl = 35.46 g of chloride
(b–a)gofAgCl = 35.46/143.34x(b–a)g of chloride or 25 ml of the given solution contains
= 35.46/143.34 ( b – a ) g of chloride
Amount of Cl- ion present in one liter of the solution
= 35.46/143.34 x( b – a )x1000/25
= 35.46 x( b – a ) x40 / 143.34 g
Similarly, the strength of NaCl solution in g/liter of the solution
= 58.46 x( b – a ) x 40 / 143.34 g
https://www.youtube.com/watch?v=p_IgKoO49iE
Chloride Test Kit manufacturer and supplier in Mumbai, JNPT, Kandla - Deen Dayal Upadhyay port, Mundra, Chennai - Ennore, Kolkata - Haldia, Buz Buz, Paradip, Visakhapatnam - Gangavaram, Fujairah, Dubai, Sharjah, Ajman, Abu Dhabi, Muscat - Oman, Nairobi Kenya, Sudan Yemen
Boiler water Chloride Test kit, CHLORICOOL WT TEST KIT, Chloride LMP Test Kit measure chloride in Boiler Water